|
An intact patch of endothelium loaded by fura-2 is shown in Fig. 1A. Scraping a few ECs by a microelectrode (Fig. 1B) increases the [Ca2+]i in the adjacent 3–4 rows of cells, the greater the distance from the injury the smaller the [Ca2+]i increase. Ca2+ signals in ECs facing the injured area are here analysed. The injury causes a quick increase in [Ca2+]i, followed by a long-lasting decay (Fig. 1C). The Ca2+ signal is present in Ca2+-free solution, but is short-lasting. When suramine, a P2Y receptor blocker, is applied in a Ca2+-free solution, no response occurs. These data suggest that injured ECs release ATP, which causes a quick [Ca2+]i increase. Ca2+ influx also occurs, allowing a long-lasting Ca2+ signal. During the decay phase ECs are able to respond to ATP (Fig. 1C) and to retain fura-2 and exclude ethidium bromide, two molecules able to cross gap junctions. Furthermore, 1) U73122 and SK&F 96365 have no effect, 2) the gap junction blocker palmitoleic acid decreases the [Ca2+]i, 3) Verapamil has no effect. When applied during Ach-evoked Ca2+ signals, 1) U73122 and SK&F 96365 inhibit Ca2+ influx, 2) palmitoleic acid has no effect. After the injury, therefore, the hemichannels of those ECs which see injured ECs close, since ECs retain fura-2 and exclude ethidium bromide. Furthermore, Ca2+ influx occurs, but neither voltage-activated Ca2+-channels nor SOCs/RACCs are involved. The effect of palmitoleic acid suggests that hemichannels have a residual permeability to Ca2+, allowing a long-lasting Ca2+ influx.
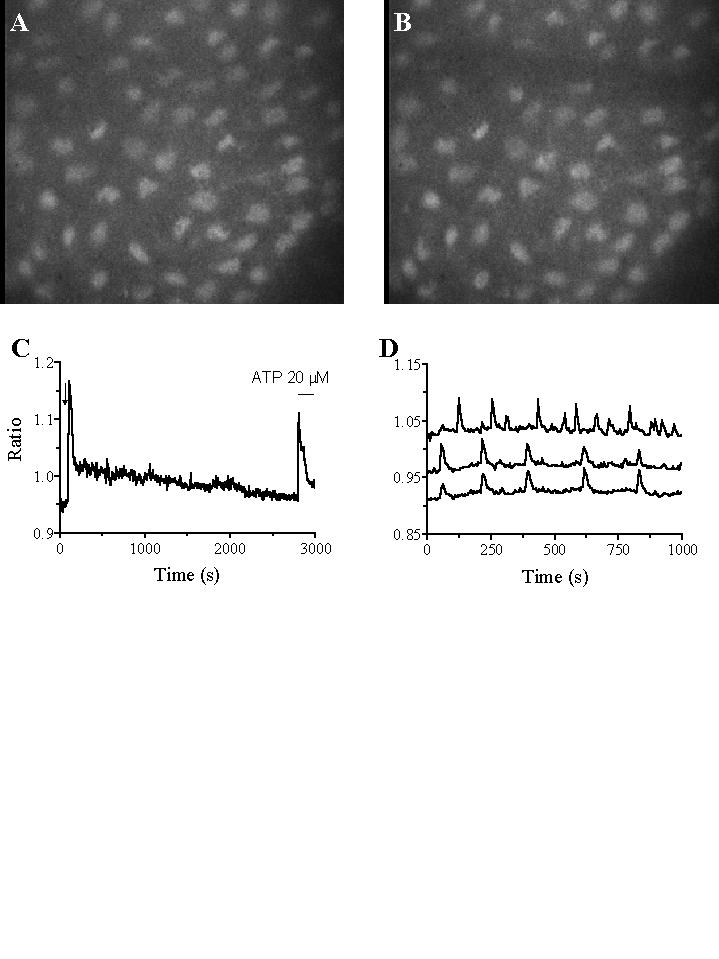
Even in the absence of intentional injury, the aortic endothelium has a number of damaged ECs, as evidenced by ethidium bromide application. About 15% of the ECs facing injured cells showed [Ca2+]i oscillations (Fig. 1D), which were blocked by extracellular Ca2+ removal, CPA and U73122 treatment, Ni2+ and La3+ application. Also heptanol, a blocker of gap junctions, inhibits [Ca2+]i oscillations. Ca2+ influx, probabily through hemichannels, occurs therefore also in such oscillating cells, but further data are required to better analyse the Ca2+ oscillation process. The increase in [Ca2+]i probabily causes NO production, which inhibits platelet activation by denuded vessel and favours EC proliferation.
|
