We generated a model (venous clip) with altered shear stress in a chicken embryo, in ovo. This results in a change in blood flow through the embryo and a concomitant cardiovascular malformation. In a large study, combining efforts from the above centers, we are gaining grip on the functional relation between physiology and genetics in the developing embryo. Part of the project is to generate physiological data (flow velocity, pressure, volume) on the performance of the chicken embryonic heart during normal and abnormal development. These data are fed into a custom made Computational Fluid Dynamics model of the beating embryonic heart in which it is possible to predict “hotspots” of shear stress changes during the various phases of contraction.
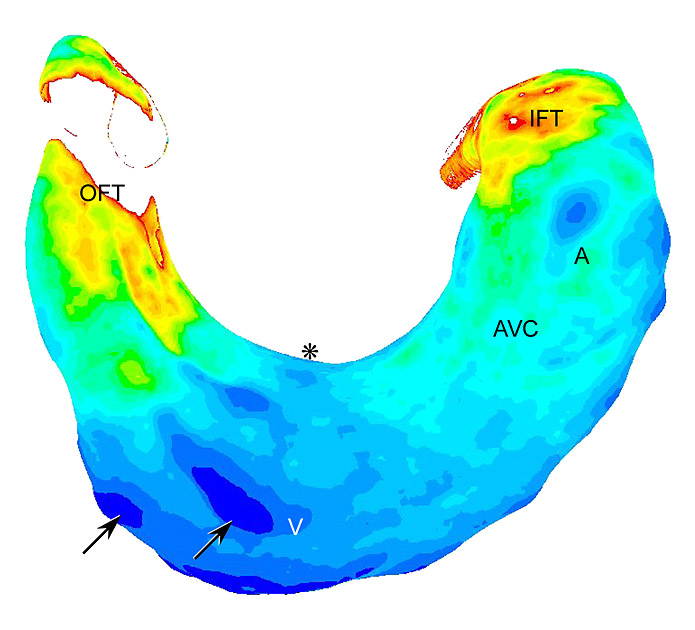
Distribution of shear stress in a CFD model of the HH15 chicken embryonic heart
More in vivo data on the distribution of flow and shear stress within an embryonic vessel will be generated with a method called microPIV (Particle Image Velocimetry). First measurements show a parabolic velocity distribution in embryonic vessels. The genetic part of the project focuses on the endothelial response to shear stress. Levels and patterns of expression of key regulatory genes, including genes of the endothelin cascade, eNOS, and KLF2 have been studied during various stages of normal development and in the venous clip model. Furthermore, shear dependent endothelial function and differentiation was determined by in vitro culture in a dynamic flow bioreactor, IHC, ISH, and QPCR. The combination of these sources of biological read-outs will provide essential information about the role of hemodynamics in cardiovascular development.
This research was supported by the Netherlands Heart Foundation NHF2000.016.