Background: Patients with severe aortic stenosis (AS) remain at risk for sudden cardiac death (SCD) even after TAVI. Pre-existing conduction abnormalities like RBBB or AV block increase the risk for new permanent pacemaker implantation after TAVI, and for SCD. Myocardial fibrosis (MF) is associated with adverse LV remodeling in AS patients and predicts cardiovascular mortality after TAVI. Moreover, MF might create vulnerable tissue substrate as source for arrhythmogenesis.
Aims: We sought to evaluate the impact of myocardial fibrosis on SCD during post-TAVI follow-up, and to link MF with ECG parameters.
Methods: We prospectively included 156 consecutive AS patients (51 women; mean age 79 years) scheduled for transfemoral TAVI (1/2017-3/2021). LV biopsies were harvested during TAVI from the basal anteroseptum. Fibrosis was assessed in Masson’s Trichrome stained biopsy sections as blue area in relation to total tissue area. ECG was analysed at baseline and discharge (ventricular pacing was excluded from analysis of conduction parameters). Patients were followed by regular telephone contact to assess incidence and causes of death. For further analysis, patients were stratified according to their fibrotic burden to MF above (MF+, n=78) or below (MF-, n=78) the median of histological MF (11.6%).
Results: During a follow-up of up to 5 years, n=20 cases of SCD occurred (n=15 in MF+, n=5 in MF-). 6/20 SCD patients had pacemakers (n=3 implanted after TAVI), none had an ICD, and 16/20 were under beta-blocker treatment. MF+ was significantly associated with higher cardiovascular mortality (HR 2.5, P=0.002), and it emerged as even stronger predictor for SCD after TAVI (HR 3.8, P=0.005, figure 1).
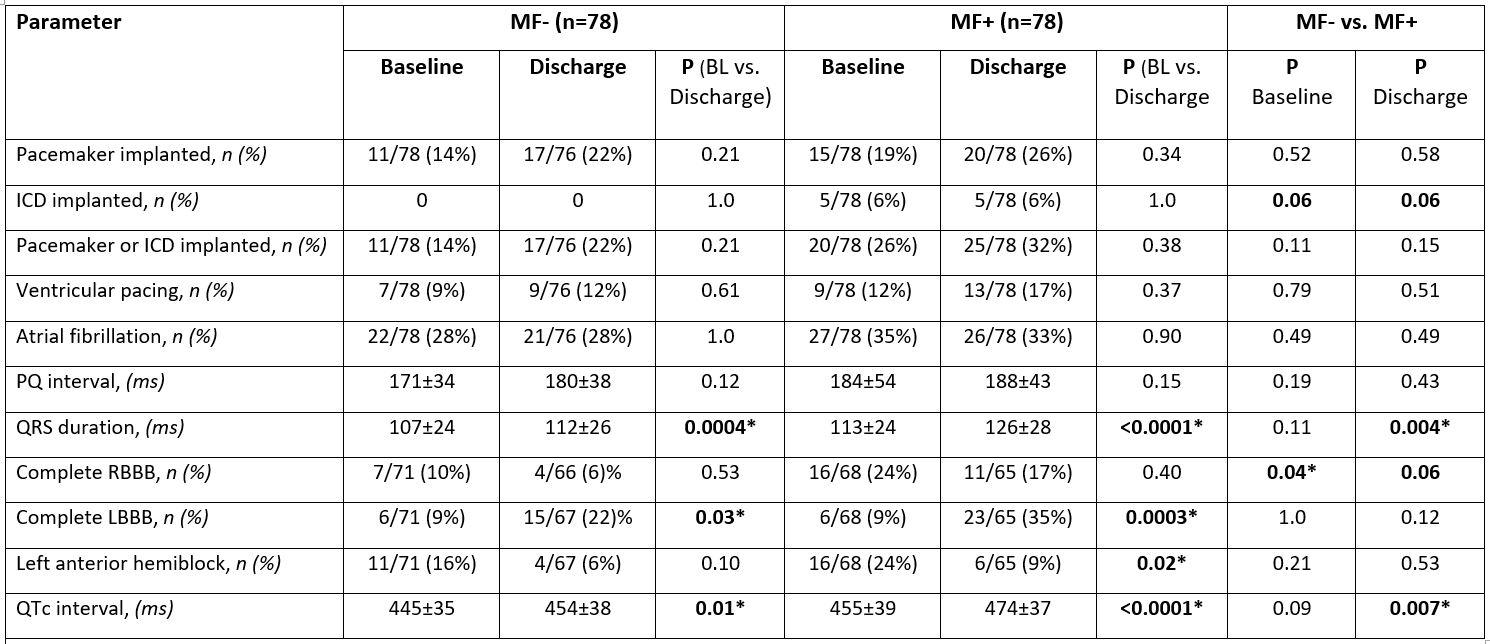
Comparison of baseline ECGs in MF+ vs. MF- patients (table 1) revealed a higher prevalence of complete RBBB in MF+ (24% vs. 10%, P=0.04), indicating a higher vulnerability for further conduction delay. Compared with baseline, TAVI lead to significant QRS prolongation at discharge in both MF groups (MF+: 113±24 vs. 126±28 ms, P<0.0001; MF-: 107±24 vs. 112±26 ms; P=0.0004). However, this prolongation was significantly more pronounced in MF+ patients, resulting in a significant longer QRS duration in MF+ (if compared with MF-) at discharge (P=0.004). Similarly, the QTc interval was significantly prolonged after TAVI in both groups if compared with baseline, but the extent of prolongation was greater in MF+, leading to a significantly longer QTc interval at discharge in MF+ (474±37 ms vs. 454±38 ms, P=0.007). Prevalence of complete LBBB at baseline was 9% in both groups, but raised to 22% in MF- vs. 35% in MF+ (P=0.12) at discharge. The incidence of new permanent pacemaker implantation did not differ between groups (n=8 in MF- and n=10 in MF+). However, since more MF+ patients had implanted devices already at baseline, the prevalence of implanted devices did nearly differ significantly 30 days after TAVI (38% in MF+ vs. 24% in MF-, P=0.08).
Conclusion: Our results suggest that, besides known procedural and anatomical factors, myocardial vulnerability as estimated by MF burden contributes to the risk of conduction disturbances after TAVI. We could link complete RBBB, QRS prolongation, and QTc interval prolongation with a high baseline MF burden – and all these parameters have previously been linked with SCD. Anti-fibrotic therapy could represent a new therapeutic strategy to improve post-TAVI outcomes.
https://dgk.org/kongress_programme/jt2023/aP905.html