Background
Long-term recurrence rates after atrial fibrillation (AF) ablation are still not satisfactory, particularly in patients with persistent AF. As catheter ablation is primarily targeting pulmonary vein (PV) ectopic activity, it is not surprising that extra-PV arrythmogenic substrate is a key determinant of arrhythmia recurrence. Against this background several studies have proposed assessment of extra-PV substrate in terms of atrial fibrosis or locally reduced conduction velocities to guide treatment decisions. However, to date no non-invasive method directly assessing electrical arrhythmogenic substrate has been established in clinical practice, and treatment decisions are commonly based on crude surrogates like AF type or left atrial size.
Here we establish and validate a novel non-invasive method based on electrocardiographic imaging (ECGi) to determine atrial arrhythmogenic substrate in terms of reduced local conduction velocities and its predictive value regarding arrhythmia recurrence after PVI. While preliminary data from this study have been presented previously, we now report the final results.
Methods and results
52 consecutive patients scheduled for AF ablation (PVI-only) and 21 healthy controls were prospectively included and received ECGi to assess left and right atrial arrhythmogenic substrate. This novel ECGi-based method uses 64 electrodes placed on the torso. Subsequently, a 3D model of the torso is acquired as an anatomical reference using a 3D reconstruction camera. A personalised 3D atrial geometry is then derived from a data base of human atria using an artificial intelligence-based algorithm. Finally, unipolar surface electrograms are projected onto the cardiac geometry and local conduction velocities are estimated.
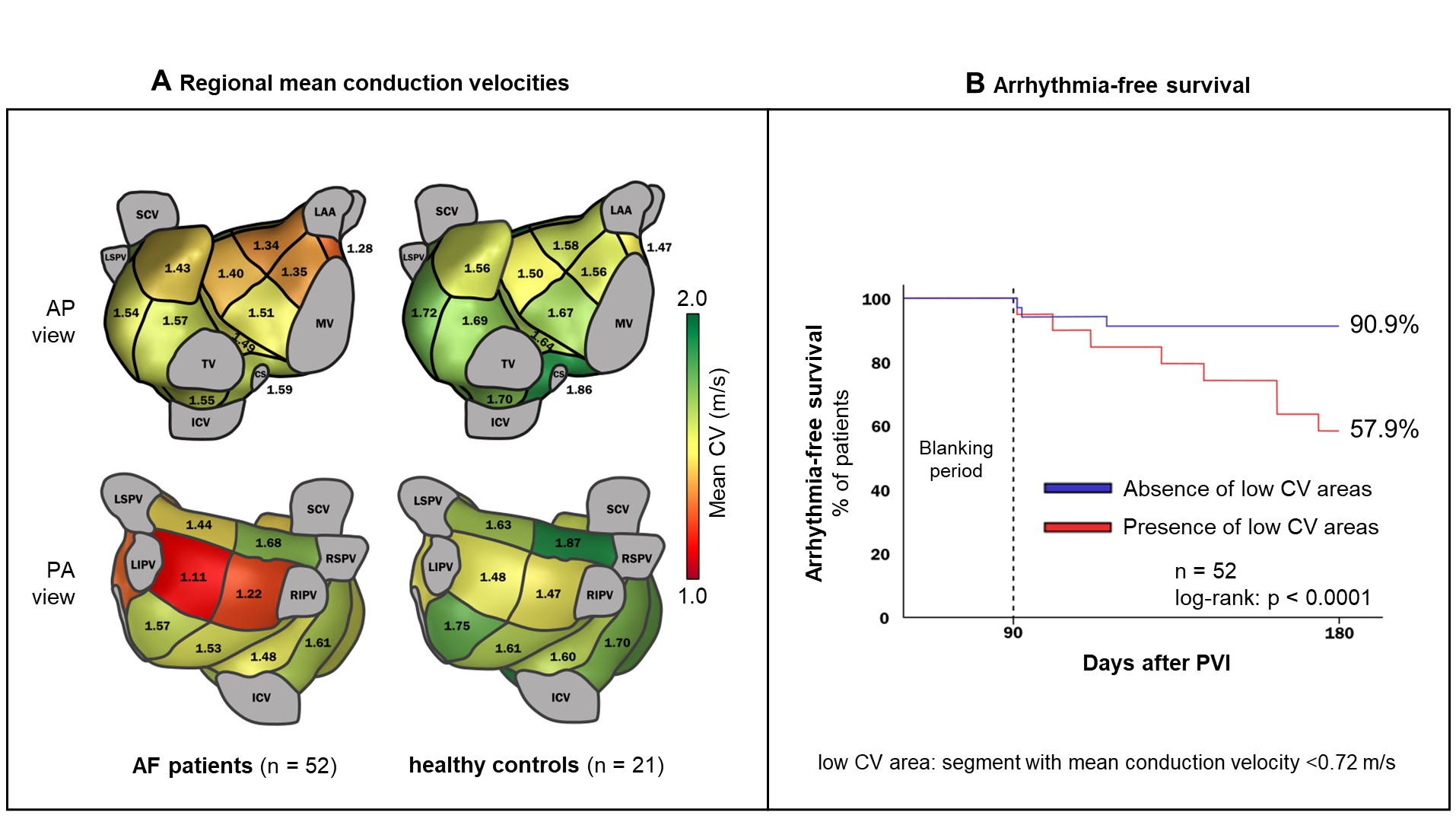
Mean ECGi-determined atrial conduction velocities were significantly lower in AF patients (1,45±0.15 m/s) than in healthy controls (1.64±0.15 m/s; p<0.0001). In a regional analysis of 19 predefined left and right atrial segments considering only the segments with the lowest average conduction velocity in each patient (indicating putative arrhythmogenic regions), differences in conduction velocities were even more pronounced (Figure 1A, 0.80±0.22 versus 1.08±0.26 m/s; p<0.0001). Multivariate logistic regression analyses combined with c-statistics including other previously proposed predictors found this mean conduction velocity of the „slowest“ segment to be the strongest predictor of arrhythmia recurrence after PVI.
A ROC analysis reveiled that a cut-off for this variable of 0.72 m/s, best discriminates PVI responders from non-responders: Patients with a mean conduction velocity of >0.72 m/s in all atrial regions showed a 6-months arrhythmia-free survival of 96%, whereas patients with one or more atrial regions with a mean conduction velocity below 0.72 m/s had a poor outcome with an arrhythmia-free survival rate of only 46% (Figure 1B).
Conclusion
This was the first study to investigate local atrial conduction velocities non-invasively and to validate their predictive value regarding outcome after PVI. In fact, the presence or absence of ECGi-determined areas of slow conduction well discriminated PVI responders from non-responders. Such non-invasive assessment of electrical arrhythmogenic substrate may guide treatment strategies and be an important step towards personalised therapy of AF.
https://dgk.org/kongress_programme/jt2023/aP866.html