Objective: Celiac disease (CeD) is an autoimmune enteropathy triggered by dietary gluten, with a prevalence of around 1%. Since many patients show heterogeneous symptoms, it is vastly underdiagnosed. Clinical studies comparing CeD patients to healthy controls have been conducted and hypothesized CeD as a cardiovascular risk factor. Herewith we aimed to explore the cardiovascular effects of CeD in a murine model, with special emphasis on endothelial dysfunction, vascular inflammation, and oxidative stress, known triggers for cardiovascular disease and its mechanistic causes.
Methodology and Results: NOD.DQ8 mice, with a genetic predisposition for CeD, were raised on a zein-based, gluten-free diet. During the experiment, mice received gavage with a peptic-tryptic digest of gliadin and were switched to a gluten-containing diet (Gliadin group). Gliadin is a protein fraction of gluten and is recognized as an antigen in CeD. A control group (Zein group) continued a gluten-free diet and received accordingly digested zein via gavage.
Infiltration by intraepithelial T-lymphocytes into the epithelium of the small intestine in the Gliadin group was shown by qIHC. Tail-cuff measurements revealed elevated blood pressure in the Gliadin group. Endothelium-dependent relaxation (ACh) was impaired in the Gliadin group compared to Zein, whereas no change in endothelium-independent relaxation (GTN) was observed, tested by isometric tension recordings. Pro-inflammatory genes (Il6, Infy, Il1b, Tnfa, Nox2), were upregulated in intestinal and aortic tissue of Gliadin mice measured by quantitative rtPCR. Flow cytometry revealed elevated CD11b+ cells in the aortic wall. In cardiac tissue, Gliadin treatment increased the oxidative stress parameters 3-nitrotyrosine (3-NT) and 4-hydroxy-2-nonenal (4-HNE) shown via dot blots. To investigate the inflammatory gut-vascular link plasma proteomics (Olink) were performed and revealed elevated levels of IL-17A, which were confirmed by plasma ELISA. The cardiovascular complications were completely reversible by a gliadin-free diet.
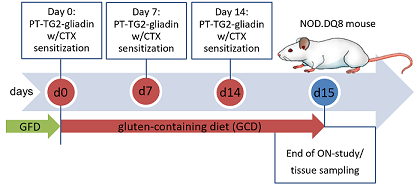
Figure 1: Treatment scheme, illustrated with the Gliadin Group.
(CTX-cholera toxin; GFD-gluten-free diet; PT-pepsin-trypsin; TG2-tissue transglutaminase)
Infiltration by intraepithelial T-lymphocytes into the epithelium of the small intestine in the Gliadin group was shown by qIHC. Tail-cuff measurements revealed elevated blood pressure in the Gliadin group. Endothelium-dependent relaxation (ACh) was impaired in the Gliadin group compared to Zein, whereas no change in endothelium-independent relaxation (GTN) was observed, tested by isometric tension recordings. Pro-inflammatory genes (Il6, Infy, Il1b, Tnfa, Nox2), were upregulated in intestinal and aortic tissue of Gliadin mice measured by quantitative rtPCR. Flow cytometry revealed elevated CD11b+ cells in the aortic wall. In cardiac tissue, Gliadin treatment increased the oxidative stress parameters 3-nitrotyrosine (3-NT) and 4-hydroxy-2-nonenal (4-HNE) shown via dot blots. To investigate the inflammatory gut-vascular link plasma proteomics (Olink) were performed and revealed elevated levels of IL-17A, which were confirmed by plasma ELISA. The cardiovascular complications were completely reversible by a gliadin-free diet.
Conclusion: In a CeD mouse model, we demonstrate that a gliadin-containing diet elevates blood pressure and impairs vascular function. CeD elevates the cardiovascular risk by intestinal inflammation that conveys to the cardiovascular system. It leads to vascular inflammation/oxidative stress, and thereby impairs endothelial function. A potential mediator might be IL-17A, whose role in other autoimmunity-based cardiovascular risk factors (e.g., psoriasis) has already been demonstrated.
https://dgk.org/kongress_programme/jt2023/aP2100.html