Background:
Sleep-disordered breathing (SDB) is frequently associated with atrial arrhythmias, but the detailed mechanisms are still unclear. We have previously reported an enhanced late Na current (late INa) in SDB, while main cardiac Na channel NaV1.5 and peak Na current were decreased, suggesting that other Na channel isoforms may be involved. Interestingly, NaV1.8-dependent signaling has been linked to pro-arrhythmic activity.
Purpose:
We hypothesized that NaV1.8 expression is increased in SDB inducing cellular Na and Ca dysregulation and subsequently arrhythmias.
Methods and Results:
Patients undergoing coronary artery bypass grafting were tested for SDB using polygraphy (apnea-hypopnea index, AHI ≥ 15/h defined SDB) and right atrial appendage biopsies were used for experimental analyses.
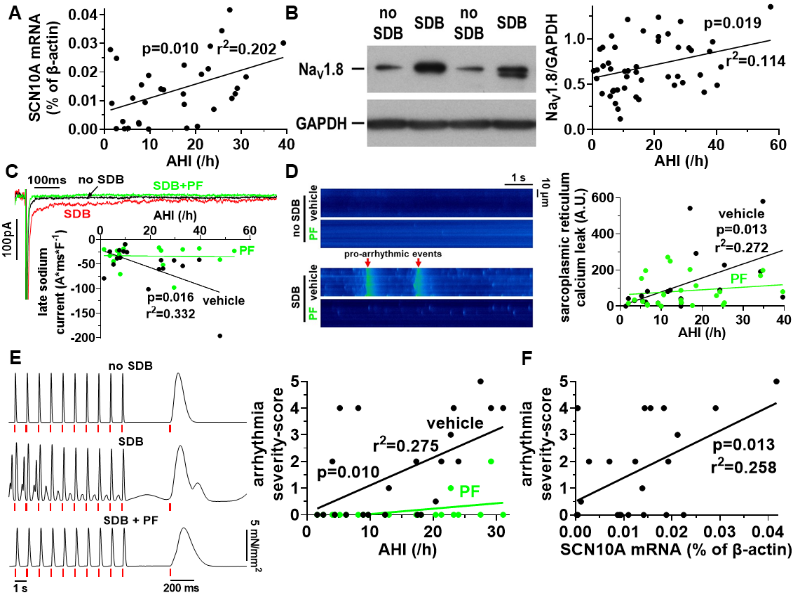
SCN10A (NaV1.8 gene) mRNA expression (qPCR) and NaV1.8 protein expression (Western blot) were found to be increased in patients with SDB and correlated with the disease severity (AHI, fig. 1A+B). The increase in NaV1.8 expression in SDB was independent of the clinical co-variates and potential confounders age, gender, body-mass index, atrial fibrillation, heart failure, diabetes mellitus, and glomerular filtration rate, as tested by multivariate linear regression analysis (p=0.020, r2=0.153).
Whole-cell voltage-clamp experiments in isolated human atrial cardiomyocytes revealed a significantly enhanced late INa in patients with SDB that correlated with the AHI (fig. 1C). Similarly, sarcoplasmic reticulum Ca leak was also increased in SDB and correlated with disease severity, as measured by confocal laser microscopy in Fluo-4 loaded cardiomyocytes. (fig. 1D). Importantly, both pro-arrhythmic mechanisms were blocked by selective NaV1.8 inhibition with PF-01247324 (PF) and the correlations with the AHI were completely abolished.
Multicellular arrhythmias were analyzed in isolated atrial trabeculae and classified by a severity score from 0 points (no arrhythmias) to 5 points (salve). In accordance with the observations on Na and Ca dysregulation, we found a significantly increased severity of arrhythmias in trabeculae from patients with SDB that correlated with the AHI (fig. 1E). Selective NaV1.8 inhibition with PF decreased the severity of trabecular arrhythmias in SDB and abolished the correlation with the AHI.
Consistently, SCN10A mRNA expression correlated significantly positive with the severity of the corresponding trabecular arrhythmias, further validating the important role of NaV1.8 in SDB (fig. 1F).
Conclusion:
Patients with SDB showed an increased atrial NaV1.8 expression resulting in a pro-arrhythmic dysregulation of cellular Na and Ca homeostasis. Selective NaV1.8 inhibition reduced pro-arrhythmic activity and blocked trabecular arrhythmias in myocardium from patients with SDB, which may have therapeutic implications.
https://dgk.org/kongress_programme/jt2023/aP1675.html