Background: The sympathetic nervous system plays an integral role in cardiac physiology. Neuromodulation is emerging as a treatment option for cardiovascular diseases, but selective intracardiac approaches are rare. Sympathetic nerves innervating the left ventricle (LV) have been proven to be amenable to transvenous catheter stimulation along the coronary sinus (CS). Therefore, the aim of the present study was to modulate LV control by selective sympathetic denervation using standard catheter ablation for intracardiac axotomy at the level of the CS.
Methods: First, the impact of epicardial CS ablation on cardiac electrophysiology was studied in a Langendorff model of murine hearts (n=10 each ablation or control). Second, feasibility of transvenous, catheter-based radiofrequency ablation by anatomically-driven axotomy across the CS was evaluated in healthy sheep (n=8).
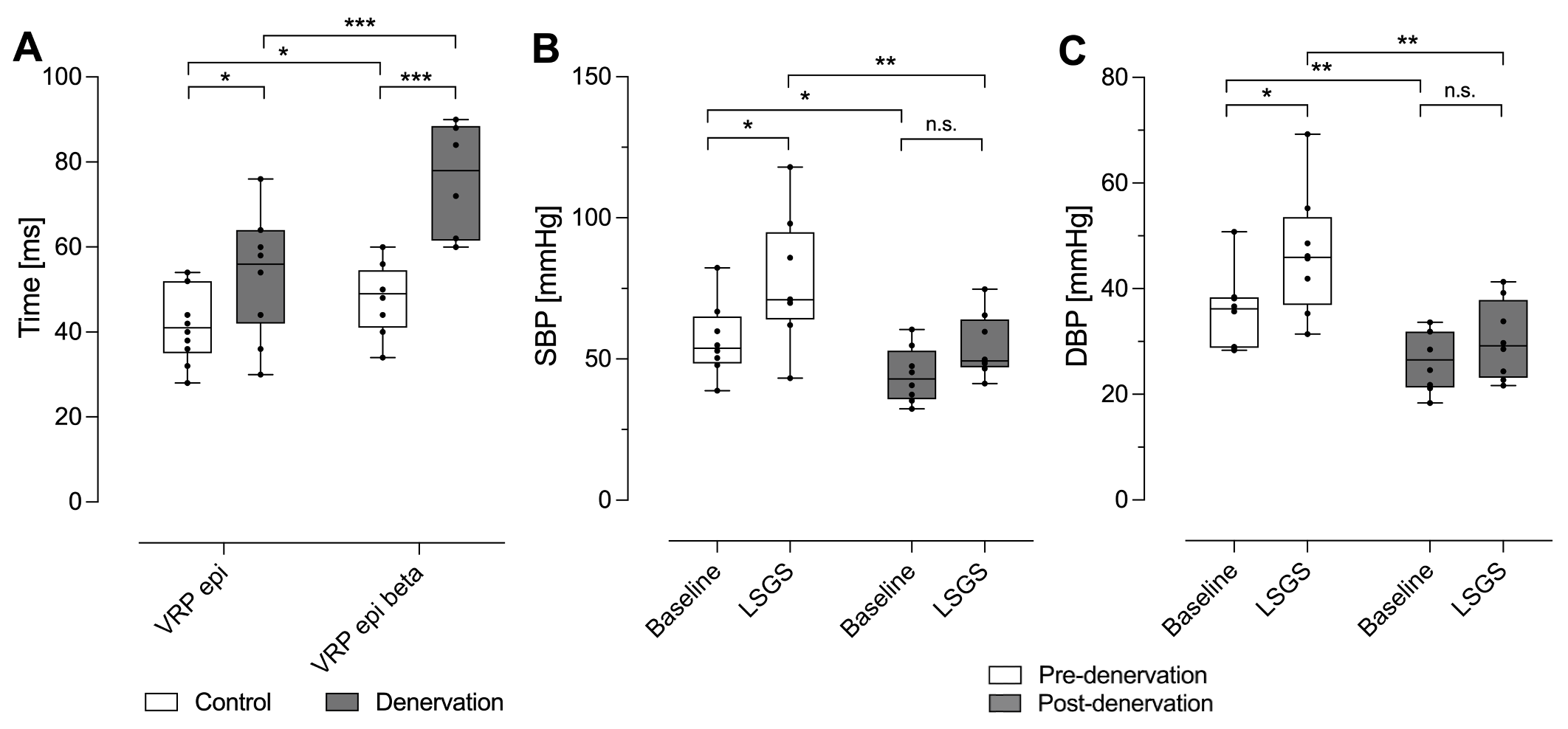
Results: CS ablation for intracardiac sympathetic axotomy prolonged epicardial ventricular refractory period (VRP) at baseline (41.8±8.4 ms vs 53.0±13.5 ms; P=0.0487) as well as after beta-blocker application (47.8±2.8 ms vs 73.1±5.0 ms; P=0.0009) and enhanced the increasing effect of beta-blockade on epicardial VRP (∆VRP 6.3±7.0 ms vs 20.0±7.5 ms; P=0.0045) in mice. Mean (1.13±0.05 m/s vs 1.00±0.02 m/s; P=0.0463) and maximum epicardial wave propagation velocity (2.90±0.16 m/s vs 2.23±0.18 m/s; P=0.0309) in the LV were faster in ablated hearts than in controls. Catheter ablation of the CS reduced systolic (SBP) (56.7±12.4 mmHg vs 44.2±9.1 mmHg; P=0.0114) and diastolic blood pressure (DBP) (35.7±2.5 mmHg vs 26.5±1.9 mmHg; P=0.0045) and diminished the blood pressure increase during left stellate ganglion stimulation (LSGS) in an ovine model (∆SBP 20.7±10.4 mmHg vs 10.2±4.6 mmHg; P=0.0186; ∆DBP 11.0±2.0 mmHg vs 3.7±0.8 mmHg; P=0.0075). Cycle length remained unchanged by LSGS, both before (baseline 652.0±21.1 ms vs LSGS 663.2±22.5 ms; P=0.7392) and after CS ablation (baseline 740.4±31.7 ms vs LSGS 772.8±10.1 ms; P=0.5632).
Conclusions: Transvenous axotomy targeting nerve fibers across the CS enables selective LV denervation resulting in acute modulation of sympathetic control.
Methods: First, the impact of epicardial CS ablation on cardiac electrophysiology was studied in a Langendorff model of murine hearts (n=10 each ablation or control). Second, feasibility of transvenous, catheter-based radiofrequency ablation by anatomically-driven axotomy across the CS was evaluated in healthy sheep (n=8).
Results: CS ablation for intracardiac sympathetic axotomy prolonged epicardial ventricular refractory period (VRP) at baseline (41.8±8.4 ms vs 53.0±13.5 ms; P=0.0487) as well as after beta-blocker application (47.8±2.8 ms vs 73.1±5.0 ms; P=0.0009) and enhanced the increasing effect of beta-blockade on epicardial VRP (∆VRP 6.3±7.0 ms vs 20.0±7.5 ms; P=0.0045) in mice. Mean (1.13±0.05 m/s vs 1.00±0.02 m/s; P=0.0463) and maximum epicardial wave propagation velocity (2.90±0.16 m/s vs 2.23±0.18 m/s; P=0.0309) in the LV were faster in ablated hearts than in controls. Catheter ablation of the CS reduced systolic (SBP) (56.7±12.4 mmHg vs 44.2±9.1 mmHg; P=0.0114) and diastolic blood pressure (DBP) (35.7±2.5 mmHg vs 26.5±1.9 mmHg; P=0.0045) and diminished the blood pressure increase during left stellate ganglion stimulation (LSGS) in an ovine model (∆SBP 20.7±10.4 mmHg vs 10.2±4.6 mmHg; P=0.0186; ∆DBP 11.0±2.0 mmHg vs 3.7±0.8 mmHg; P=0.0075). Cycle length remained unchanged by LSGS, both before (baseline 652.0±21.1 ms vs LSGS 663.2±22.5 ms; P=0.7392) and after CS ablation (baseline 740.4±31.7 ms vs LSGS 772.8±10.1 ms; P=0.5632).
Conclusions: Transvenous axotomy targeting nerve fibers across the CS enables selective LV denervation resulting in acute modulation of sympathetic control.
Figure: Selective sympathetic denervation acutely modulates LV control.
A, Electrophysiological parameters for the denervation as well as the control group are shown. Following LV denervation, epicardial VRP at baseline and after beta-blocker application was prolonged. The increasing effect of beta-blockade on epicardial VRP was enhanced. B-C, Baseline and maximum values during LSGS of B, SBP and C, DBP before and after LV denervation are depicted. Before denervation, LSGS caused an increase in SBP and DBP. After denervation, SBP and DBP as well as the increasing effect of LSGS on both parameters were reduced.
A, Electrophysiological parameters for the denervation as well as the control group are shown. Following LV denervation, epicardial VRP at baseline and after beta-blocker application was prolonged. The increasing effect of beta-blockade on epicardial VRP was enhanced. B-C, Baseline and maximum values during LSGS of B, SBP and C, DBP before and after LV denervation are depicted. Before denervation, LSGS caused an increase in SBP and DBP. After denervation, SBP and DBP as well as the increasing effect of LSGS on both parameters were reduced.
Data are presented as box plots (minimum to maximum). DBP, diastolic blood pressure; LSGS, left stellate ganglion stimulation; LV, left ventricular; n.s., not significant; SBP, systolic blood pressure; VRP epi, epicardial ventricular refractory period; VRP epi beta, epicardial ventricular refractory period after beta-blockade. *P<0.05; **P<0.01; ***P<0.001.
https://dgk.org/kongress_programme/jt2022/aV944.html