Background:
Mitral valve edge-to-edge repair (TEER) is an established therapeutic approach for relevant mitral regurgitation (MR) to alleviate symptoms and improve quality of life. Yet, the population of patients with relevant MR who stand to gain optimal benefit from TEER remains to be determined. Prior to TEER, a heart-team approach with interdisciplinary decision-making is mandatory integrating the patient profile including relevant co-morbidities as well as the application of established surgical risk scores. Whether alternative risk prediction is more suitable for this frailty patient cohort burdened with various co-morbidities has not been examined in detail. A simplified approach may be achieved by using the C-Reactive Protein to Albumin Ratio (CAR), but its value in TEER is unclear.
Methods:
This single-center, retrospective study thought to determine long-term prognostic accuracy of different risk scores in patients with relevant MR undergoing TEER. From September 2008 until January 2020, n=316 patients with a median follow-up time of 5.81 years were included. The primary outcome measure was defined as all-cause mortality. ROC analysis was conducted for the identification of the optimal CAR threshold, subsequently dichotomizing patients into two groups (CAR ≤0.4 and CAR>0.4) estimating their long-term event rate using the Kaplan-Meier method. In addition, we evaluated the prognostic value of CAR compared to two conventional surgical risk scores (logistic EuroSCORE and Society of Thoracic Surgeons [STS] risk score) using C-Index analysis.
Results:
Among 316 high-risk patients undergoing TEER (mean age 75.6 ± 8.2 years, 61.7% male, median logistic EuroSCORE 19.9% [interquartile range 11.7; 31.6], median STS Score 3.8% [interquartile range 2.2; 5.7]), 176 (55.7%) patients showed CAR ≤0.4. Patients with elevated CAR (>0.4) predominantly suffered from a higher burden of co-morbidities, such as peripheral artery disease (p=0.001), chronic obstructive pulmonary disease (p=0.044), and chronic kidney disease (p=0.015). Consequently, these patients had significantly higher logistic EuroSCORE and STS Score than patients with CAR ≤0.4 (logistic EuroSCORE p=0.002; STS Score p<0.001). Stratification according to the CAR threshold of 0.4 led to significant differences in the Cumulative Incidence curves (p<0.001). In addition, log-rank test revealed a superior risk stratification of the simplified CAR approach compared to the established surgical risk scores (Figure 1). This effect consequently reflects in a higher adjusted C-Index for CAR (0.608) compared to logistic EuroSCORE (0.502; p<0.001) and STS Score (0.498; p<0.001).
Conclusions:
Our data provide first evidence that alternative risk prediction using CAR allows a feasible and easy-to-use risk prediction regarding all-cause mortality in a real-word TEER cohort presenting with advanced age, a high proportion of frailty and numerous co-morbidities. Alternative risk prediction in TEER patients should be studied as established surgical risk scores were developed for an entirely different patient population and seem to demonstrate limited applicability in patients scheduled for TEER.
Figure 1. Cumulative Incidence curves according to CAR, EuroSCORE and STS Score.
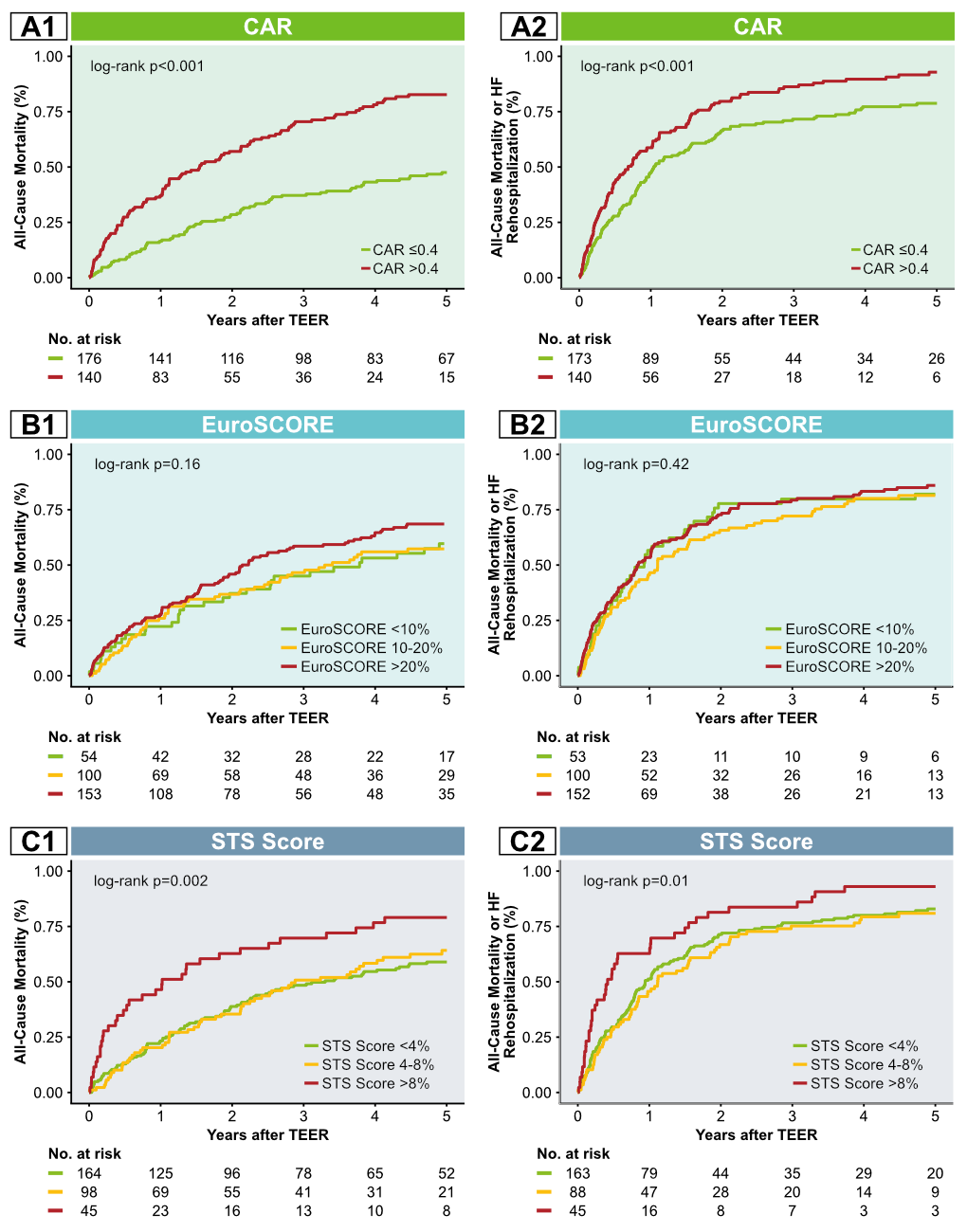
Cumulative incidence curves for the primary endpoint according to CAR (A1), EuroSCORE (B1) and STS Score (C1) and respectively for the secondary endpoint of All-Cause Mortality or Heart Failure Rehospitalization (A2, B2 and C2).
Figure 1. Cumulative Incidence curves according to CAR, EuroSCORE and STS Score.
Cumulative incidence curves for the primary endpoint according to CAR (A1), EuroSCORE (B1) and STS Score (C1) and respectively for the secondary endpoint of All-Cause Mortality or Heart Failure Rehospitalization (A2, B2 and C2).
https://dgk.org/kongress_programme/jt2022/aV1704.html