DOI DOI https://doi.org/10.1007/s00392-021-01843-w
Background: Ultra-high-density mapping (UHDM) enables detailed analysis of arrhythmia mechanisms, but has not been used before to improve our understanding of atrial reentrant circuit conduction velocity (CV) patterns in adult congenital heart disease (ACHD).
Purpose: To investigate ACHD circuit mechanisms using UHDM.
Methods: ACHD patients with recurrent supraventricular tachycardia who underwent UHDM-guided catheter ablation between February 2017 and September 2020 were enrolled in this retrospective dual-center study. Circuit maps consisting of 10 isochrones of activation were created and CVs for each isochrone calculated. Tachycardias were classified as “extensive” or “minimal” slowing when the number of isochrones <0.3 m/s was >5 or ≤5, respectively (50th percentile). Circuit heterogeneity was determined by the coefficient of variability.
Results: 81 patients (42.4±16.6 years, 56% male) underwent 85 procedures targeting 136 supraventricular tachycardias (60% intra-atrial reentrant tachycardia, 14% multiple-loop). Circuits with minimal vs extensive slowing featured longer pathway length (126.3±34.6 mm vs 64.8±19.3 mm; P<.001), shorter signal duration (78.8±28.5 ms vs 97.1±28.3 ms; P=.013) and faster average CV (0.46±0.10 m/s vs 0.23±0.07 m/s; P<.001). The number of slow isochrones was associated with the coefficient of variability (P<.001), indicating a more heterogeneous CV pattern for tachycardias with extensive slowing (0.89±0.22 vs 0.61±0.17; P<.001). Circuits using anatomical obstacles showed less slowing (4.5±2.4 vs 6.1±1.8; P=.005) and heterogeneity (coefficient 0.66±0.19 vs 0.84±0.25; P=.003) than those based on surgically-induced obstacles; with Tetralogy of Fallot (TOF) displaying the slowest and most heterogeneous patterns compared to all other patients (coefficient 1.03±0.11 vs 0.71±0.03; P<.001). Over a median of 369 days (IQR 224–534) arrhythmia-free survival was better in patients presenting with tachycardias with homogeneous vs heterogeneous CV (90% vs 57%; P=.038).
Conclusion: UHDM-guided CV analysis allows identification of 2 atrial reentry patterns in ACHD. These vary by both central obstacle and congenital lesion with surgically-acquired substrates and TOF showing the most extensively slow and heterogeneous circuits. CV analysis may predict arrhythmia recurrence in this population.
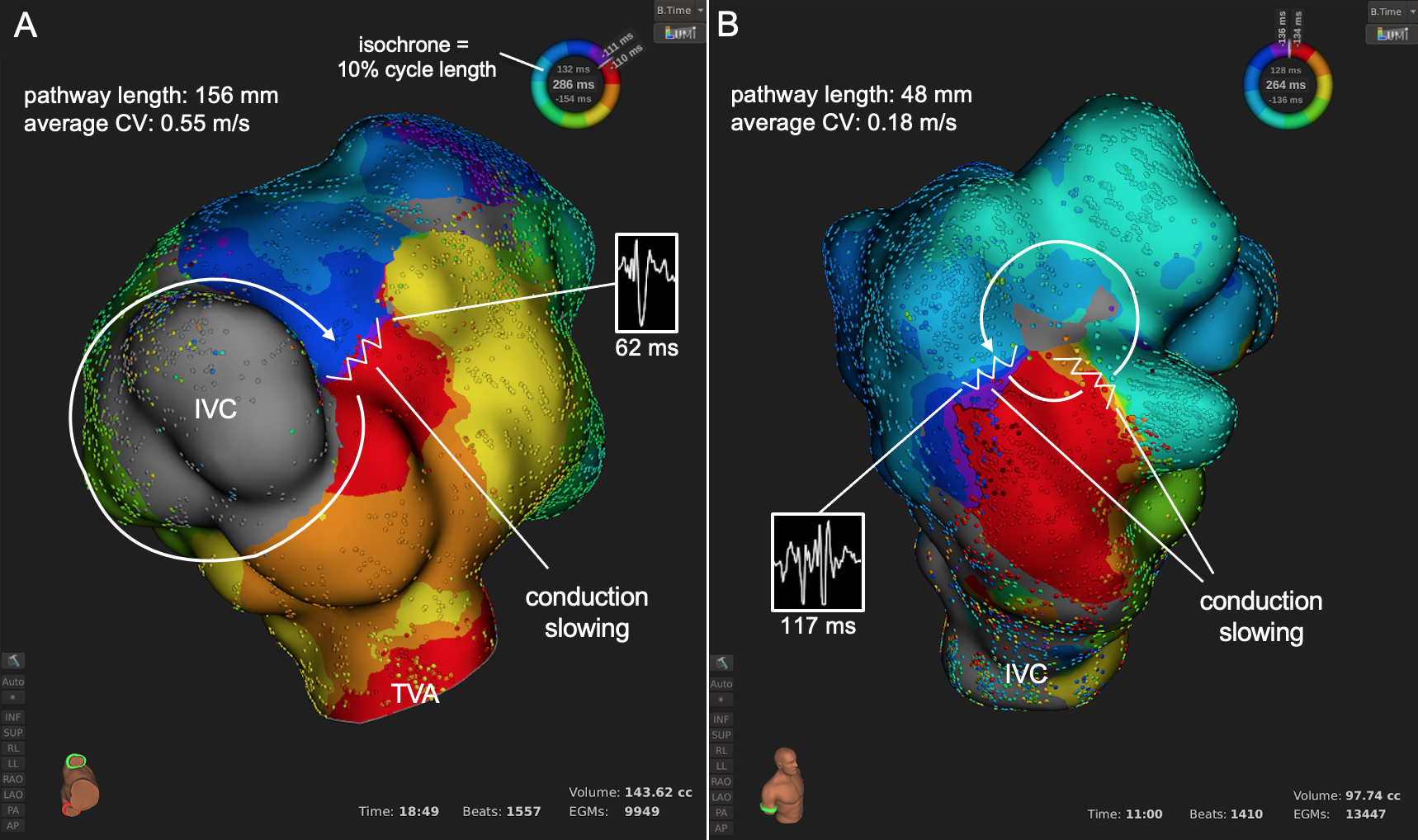
Figure: Representative isochronal maps depicting the major circuit subtypes.
Figure: Representative isochronal maps depicting the major circuit subtypes.
(A) Reentry around an anatomic obstacle (IVC) with minimal slowing at a single critical isthmus in a patient with a hypoplastic right ventricle after 1.5 ventricle repair with Glenn anastomosis.
(B) Reentry around a surgically-induced free wall scar with extensive slowing in a patient with repaired TOF. Although the slowest portion passed through an inaccessible area near a lead insertion site, another isthmus was localized inferolateral and successfully ablated.
IVC, inferior vena cava; TVA, tricuspid valve annulus
https://dgk.org/kongress_programme/jt2021/aV142.html