DOI DOI https://doi.org/10.1007/s00392-021-01843-w
Objective:
Patients with sleep-disordered breathing (SDB) frequently develop atrial fibrillation (AF). We have shown previously that enhanced late Na current (late INa) caused by increased Ca/Calmodulin-dependent kinase II (CaMKII) phosphorylation of NaV1.5 contributes to atrial pro-arrhythmic activity in patients with SDB. Interestingly, recent data suggests that NaV1.8-dependent late INa may be involved in AF development.
Purpose:
We hypothesized that NaV1.8 expression and NaV1.8-dependent pro-arrhythmic signaling may be increased in patients with SDB.
Methods and Results:
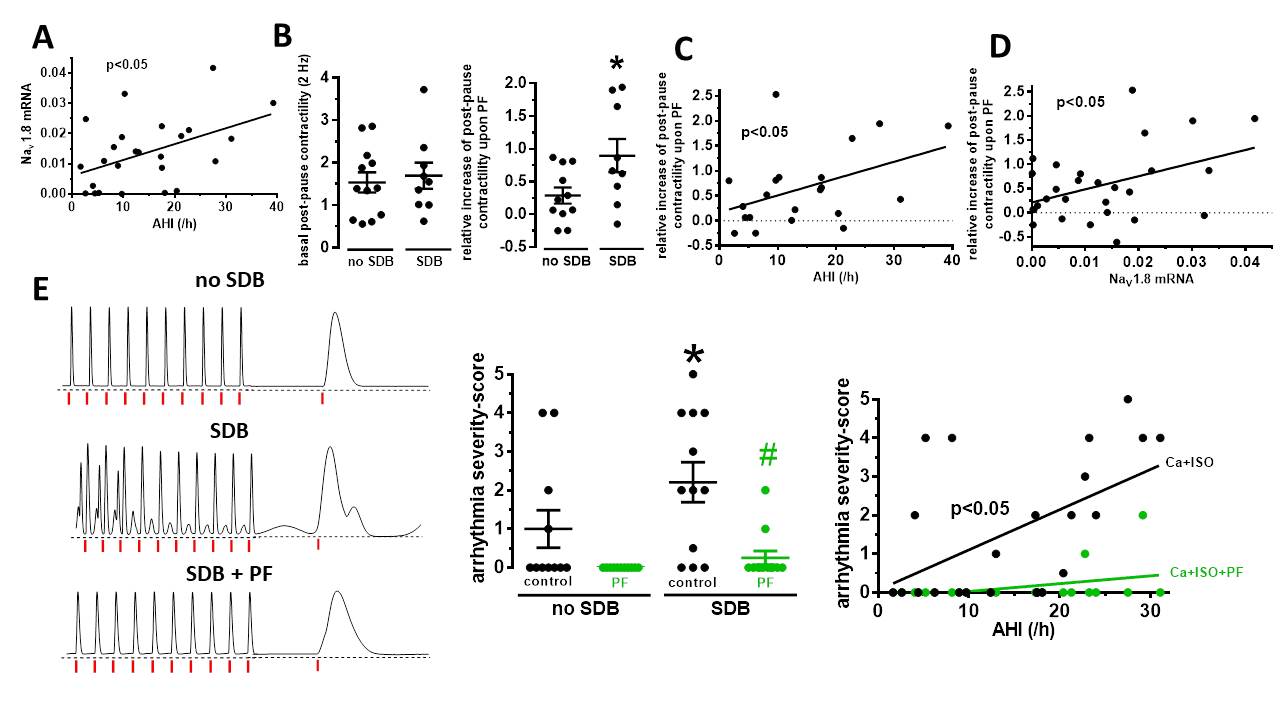
Right atrial appendage biopsies were collected from 29 patients undergoing elective coronary artery bypass grafting. They were monitored for SDB by polygraphy during the night before surgery and SDB was defined by an apnea-hypopnea index (AHI) ≥15/h. Regular contractions were elicited in isolated atrial trabeculae by electrical field stimulation (at 1 Hz) to monitor contractility and pro-arrhythmic activity. Thereafter, atrial myocardium was used for qPCR, which enabled us to correlate functional and expression data for each individual patient. Interestingly, we observed an increase in NaV1.8 mRNA expression in patients with SDB that correlated significantly positive with disease severity (i.e. AHI, p=0.02, R²=0.22, fig. 1A). Post-pause contractility (at 2 Hz, normalized to pre-pause), was used to estimate sarcoplasmic reticulum Ca leak. Intriguingly, selective inhibition of NaV1.8 by exposure to PF-01247324 (PF, 1 µM, 30 min) resulted in a significantly larger relative increase in post-pause contractility of 0.90±0.25 (mean ± SEM) in patients with SDB compared to 0.29±0.12 in patients without SDB (p=0.04, fig. 1B) leading to a significant positive correlation with the AHI (p=0.047, R²=0.19, fig. 1C). Moreover, the PF-dependent relative increase in post-pause contractility correlated significantly positive with NaV1.8 mRNA expression (p=0.03, R²=0.17, fig. 1D). In accordance, relaxation time to 90% (RT90) was significantly accelerated by exposure to PF (in ms) from 142.04±4.68 to 127.15±5.01 (p<0.05) in patients with SDB (N=14), but no effect was observed in patients without (N=15). Trabecular arrhythmias were induced by 100 nM isoproterenol at [Ca]o of 3.5 mmol/L and arrhythmia severity was graded by a score from 0 points (no arrhythmias) to 5 points (salve). Interestingly, mean arrhythmia severity-score was significantly higher in patients with SDB compared to those without SDB (2.21±0.52 vs. 1.00±0.49, p=0.03) and could be significantly reduced by selective NaV1.8-blockade with PF to 0.25±0.18 (p=0.0008, fig. 1E). In accordance, the significant positive correlation between the severity of trabecular arrhythmias and the AHI (p=0.01, R²=0.28) was completely abolished upon PF (fig. 1E).
Conclusion:
NaV1.8 expression is increased in patients with SDB. Selective inhibition of NaV1.8 reduced atrial pro-arrhythmic activity of SDB patients, which may have therapeutic implications.
https://dgk.org/kongress_programme/jt2021/aP251.html