DOI DOI https://doi.org/10.1007/s00392-021-01843-w
Background: Coronary complications are an important determinant for sudden cardiac death and infarction following arterial switch operation (ASO) in patients with transposition of great arteries (TGA). We therefore sought to assess the role of coronary problems and myocardial fibrosis on myocardial blood (MBF) flow as assessed by cardiac magnetic resonance imaging (CMR) in patients with TGA following ASO.
Methods: In this prospective single centre analysis patients with TGA status post ASO and controls without known cardiovascular disease were studied with 3 Tesla cine CMR analysis. MBF was assessed at rest (rMBF) and under adenosine stress (sMBF) and extracellular volume fraction (ECV) was assessed by T1 mapping. We used a generalized linear model of location, shape and scale (GAMLSS) with sMBF as dependent variable, and ECV, TGA pathology, and coronary type as predictors. To account for the observed heteroscedasticity, we assumed that the variance of stress MBF changes with ECV.
Results: In the current study 113 TGA (15.2 [IQR 9.9, 20.8] years, 33% female) and 68 control patients (12.8 [IQR 7.8, 22.8] years, 50% female) were included. Of those 11 patients (10%) had coronary problems in terms of stenosis or occlusion and 16 patients had additional valvular pathologies (14%).
While rMBF was comparable between TGA and control patients (median 0.89 [IQR 0.77, 1.04] vs. 0.94 [0.80, 1.10] ml/min/g, p=0.27), sMBF (2.63 [2.11, 3.24] vs. 3.30 [2.94, 3.86] ml/min/g, p<0.001) and myocardial perfusion reserve (2.63 [2.11, 3.24] vs. 3.55 [2.92, 4.36], p<0.001) were reduced in TGA patients. ECV was higher in TGA patients as compared to controls (28.2 [26.0, 33.6] vs. 26.8 [25.6, 27.4]%, p=0.028).
The GAMLSS analysis revealed that both increased ECV as well as the presence of coronary problems were predictors of a decreased sMBF (p<0.001 for both), while rMBF as well as the presence of valvular pathologies were predictors for a higher sMBF (Table 1).
Conclusion: TGA patients are marked by comparable rMBF but lower sMBF and higher ECV as compared to healthy controls. The presence of coronary problems, as well as the extent of myocardial fibrosis as assessed by ECV are predictors of sMBF in TGA patients in a GAMLSS model. Problematic coronary reimplantation as well as progressive myocardial fibrosis might be hallmarks and determinants of impaired vasoreactivity among TGA patients following ASO.
Table 1
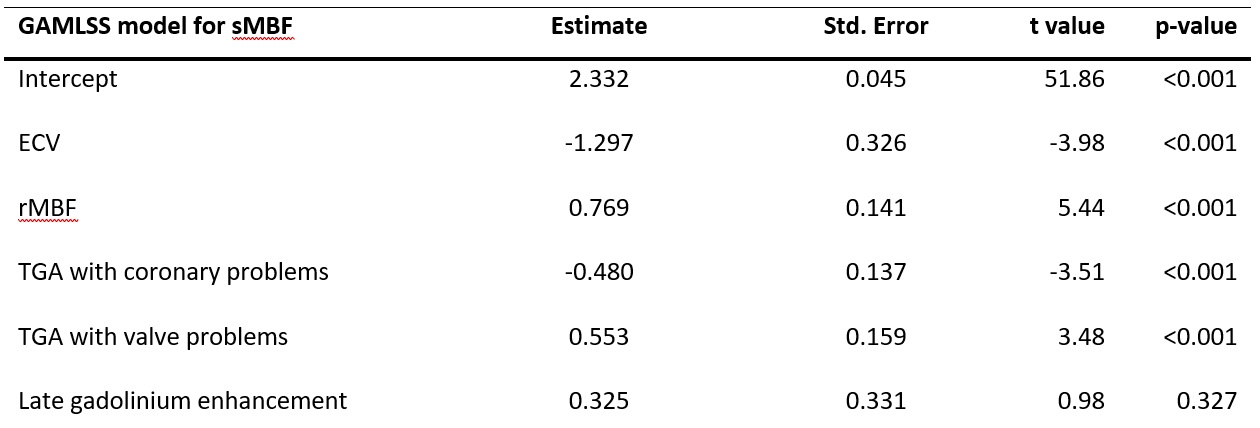
Table 1
https://dgk.org/kongress_programme/jt2021/aP1016.html