Background: Calcific aortic valve disease (CAVD) is the most common heart valve disease during adulthood and is associated with high morbidity and mortality, especially in a symptomatic stage.
During disease initiation, endothelial dysfunction leads to lipid accumulation in the interstitium and local inflammation promoting pro-calcifying pathways in interstitial cells.
Suppressor of Cytokine Signaling 3 (SOCS3) is one important regulator of inflammation. It promotes the polarization of macrophages to either a classical (M1) or an alternative subtype (M2). In the pathogenesis of atherosclerosis, SOCS3 has been shown promote plaque development and calcification.
Besides inflammation and calcification, heterotopic bone formation contributes to disease progression in CAVD and as SOCS3 is one of the main regulators of physiological bone formation, it could play a critical role in the progression of CAVD.
Purpose: Our aim is to investigate the impact of SOCS3 on the pathogenesis of CAVD. We hypothesize that SOCS3 contributes to disease initiation and progression by promoting calcification of valvular interstitial cells (VICs), endothelial to mesenchymal transition (EndMT) of valvular endothelial cells (VECs) as well as the polarization macrophages to an inflammatory phenotype.
Methods and Results: In initial experiments we aimed to evaluate the expression of SOCS3 in stenotic and non-stenotic aortic valves. Gene expression analysis and immunofluorescence (IF) staining of aortic valves from patients undergoing surgical aortic valve replacement show that SOCS3 is expressed in both stenotic and non-stenotic aortic valves.
IF staining revealed co-localization of SOCS3 with Vimentin, which is an interstitial cell marker.
We can show a similar expression pattern in IF staining of SOCS1, which is known to regulate macrophage polarization together with SOCS3 (Fig. 1). Staining of the macrophage marker CD68 shows its expression in calcified aortic valve cusps.
To further validate our findings, we stimulated human VICs with an osteogenic or a procalcifying medium to promote calcification in vitro. The upregulation of RUNX2 and BMP2 verified successful osteogenic differentiation of the cells. SOCS3 as well as SOCS1 were upregulated in differentiated VICs, especially after 7 days of stimulation.
To investigate the role of SOCS3 during endothelial to mesenchymal transition (EndMT) the cell culture medium was supplemented with TNF-α or TGF-β and IL-1β. After 7 and 14 days of cultivation, a downregulation of endothelial cell markers Von-Willebrand Factor and NOS3 and the upregulation of Vimentin and the EndMT-marker SNAI2 was noted. Simultaneously,a significant up-regulation of SOCS1 and SOCS3 was detected after 7 days of stimulation.
Conclusion: SOCS3 is expressed in human aortic valves. In vitro models show an upregulation of SOCS3 during calcification and EndMT. Ongoing experiments will help to investigate the potential role of SOCS3 in CAVD development.
Fig 1: Immunofluorescence staining of formalin-fixed, paraffin-embedded human aortic valve leaflets, explanted due to CAVD
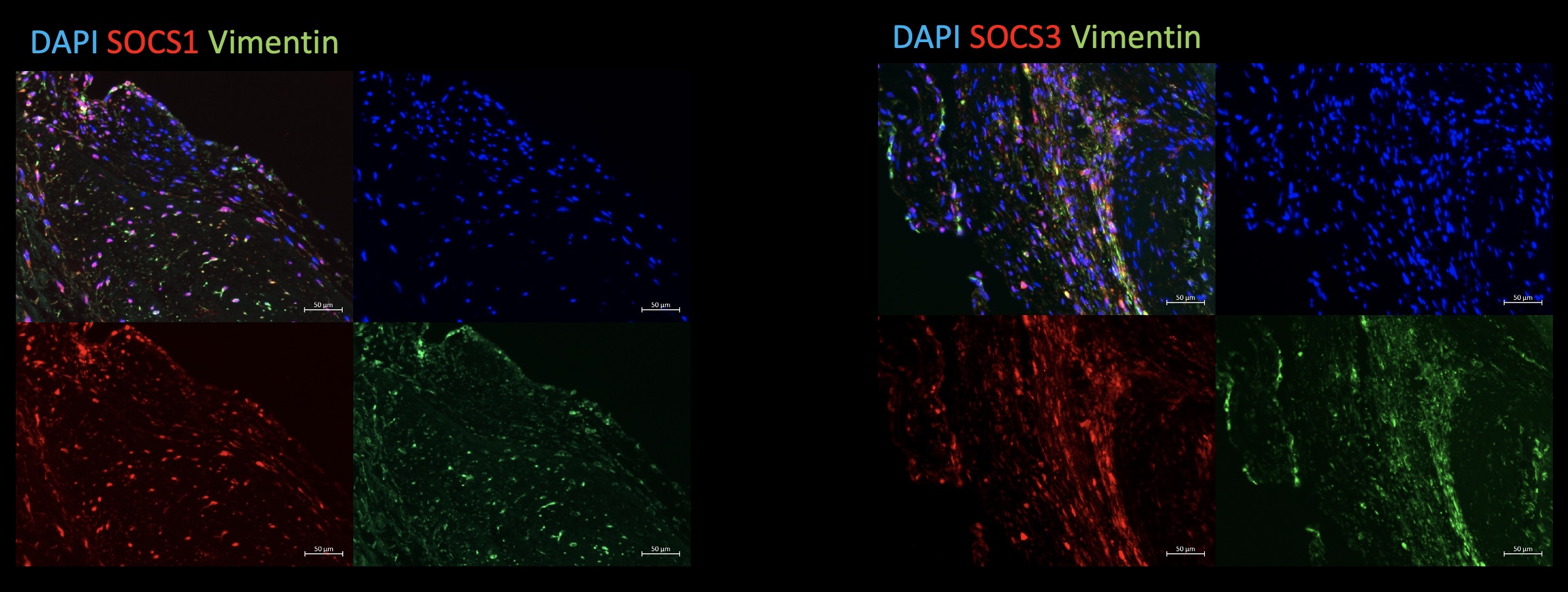
Fig 1: Immunofluorescence staining of formalin-fixed, paraffin-embedded human aortic valve leaflets, explanted due to CAVD
https://dgk.org/kongress_programme/ht2022/aBS681.html