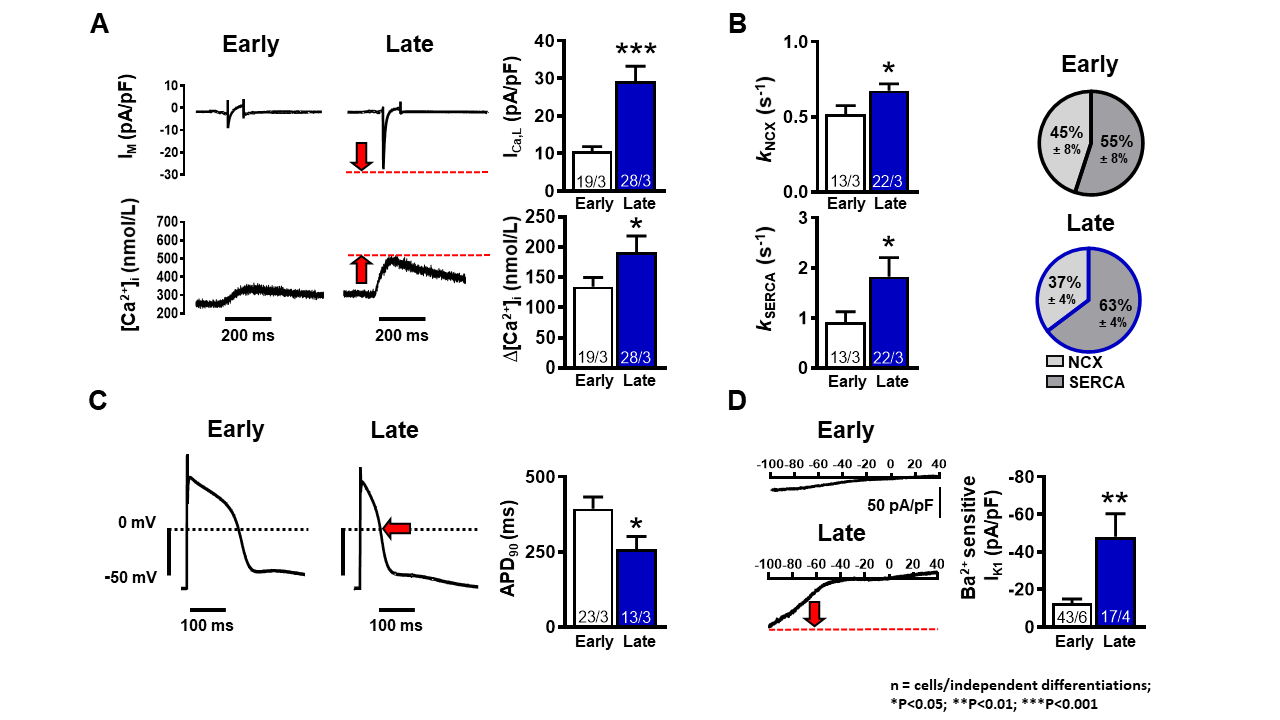
Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) have emerged as an attractive platform for personalized medicine and preclinical cardiotoxicity testing. However, the usage of hiPSC-CMs remains limited due to their immature phenotype. This study was aimed at characterizing the maturation of electrical function and Ca2+-handling in hiPSC-CM over extended monolayer culture. Simultaneous L-type Ca2+ current (ICa,L) (whole-cell voltage-clamp) and [Ca2+]i measurements (epifluorescence, Fluo-3 AM) revealed a 3-fold increased ICa,L density and enhanced systolic [Ca2+]i transient (CaT) amplitude in ‘late’ (>50 days post differentiation) vs ‘early’ (<50 days post differentiation) hiPSC-CMs (134.30±15.49 nmol/L vs 191.60±26.81 nmol/L, n/N=19/3 vs 28/3, P<0.05) (Figure, Panel A). Additionally, we observed ~30% higher NCX and ~50% higher SERCA rate constants (kNCX and kSERCA respectively) obtained from the systolic and caffeine-induced CaT decay (Panel B), suggesting an enhanced Ca2+-removal mechanisms in late cells compared to early cells. The contribution of SERCA activity to diastolic Ca2+ removal appeared to be increased in late vs early hiPSC-CMs (Panel B). Action potentials recorded in current-clamp configuration showed ~65% reduction in action potential duration in late vs early hiPSC-CM (Panel C). Concurrently, we observed an increased inward-rectifying K+ current (IK1) density in late cells compared to early cells ( 48.00±12.30 pA/pF vs 12.63±2.28 pA/pF, n/N=14/4 vs 43/6, P<0.01) (Panel D). In conclusion, electrophysiological development of 2D cultured hiPSC-CMs is characterized by an increased dominance of SERCA activity in the process of cytosolic Ca2+ removal. Extended hiPSC-CM monolayer culture drives functional expression of IK1, contributing to maturation-dependent AP shortening.
https://dgk.org/kongress_programme/ht2022/aBS671.html