Objectives: The complement system and its anaphylatoxin C5a as part of the innate immune system play a critical role in a variety of inflammatory conditions. Recently the presence of the C5a receptor 1 (C5aR1) has been detected on platelets. There is new evidence suggesting a rather unknown role of the C5aR1-axis: both global and platelet specific inhibition showed to increase neovascularization after experimental hindlimb ischemia.
We aimed to investigate if C5aR1 deficiency would increase neovascularization in myocardial ischemia and improve heart function.
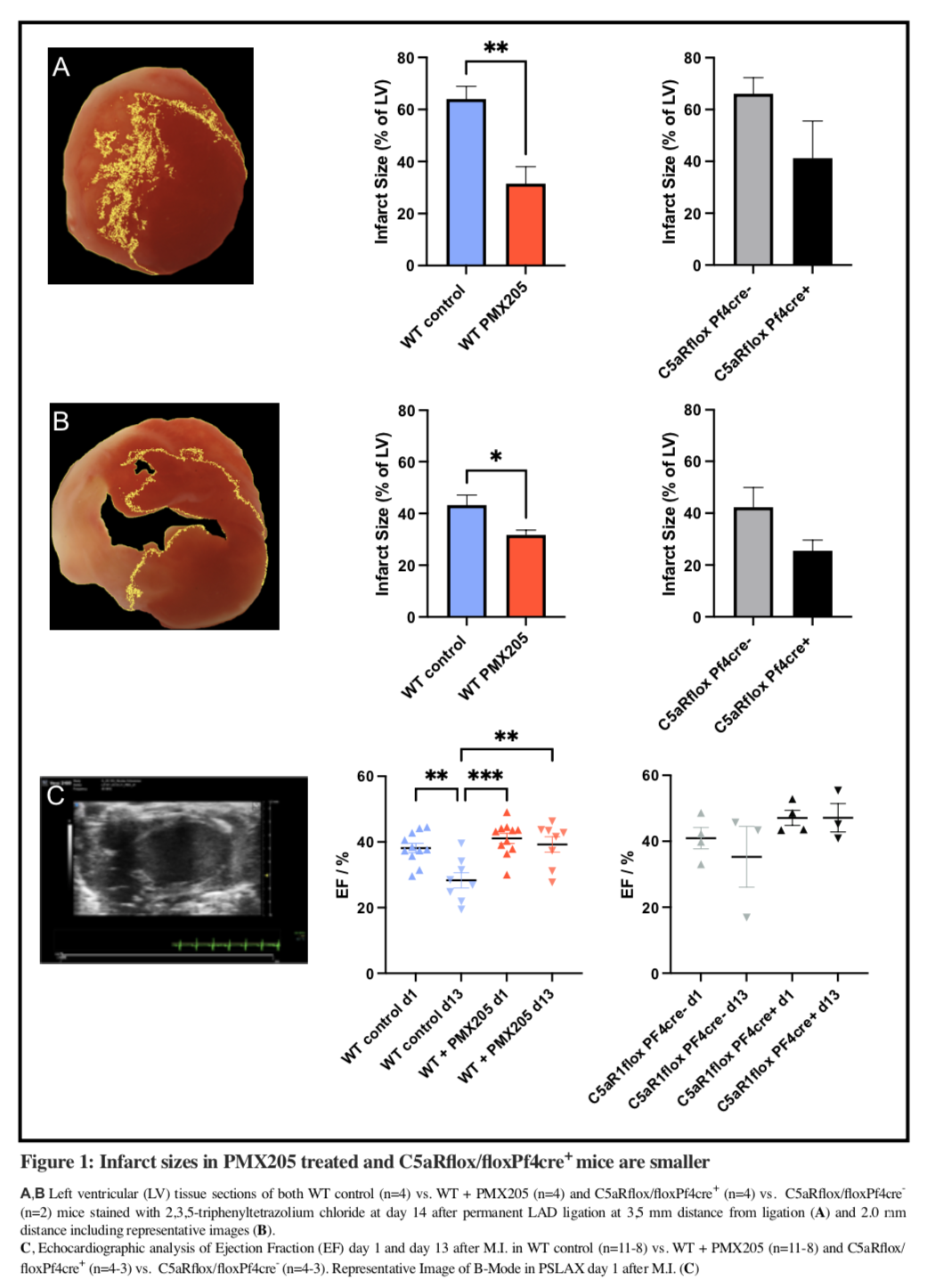
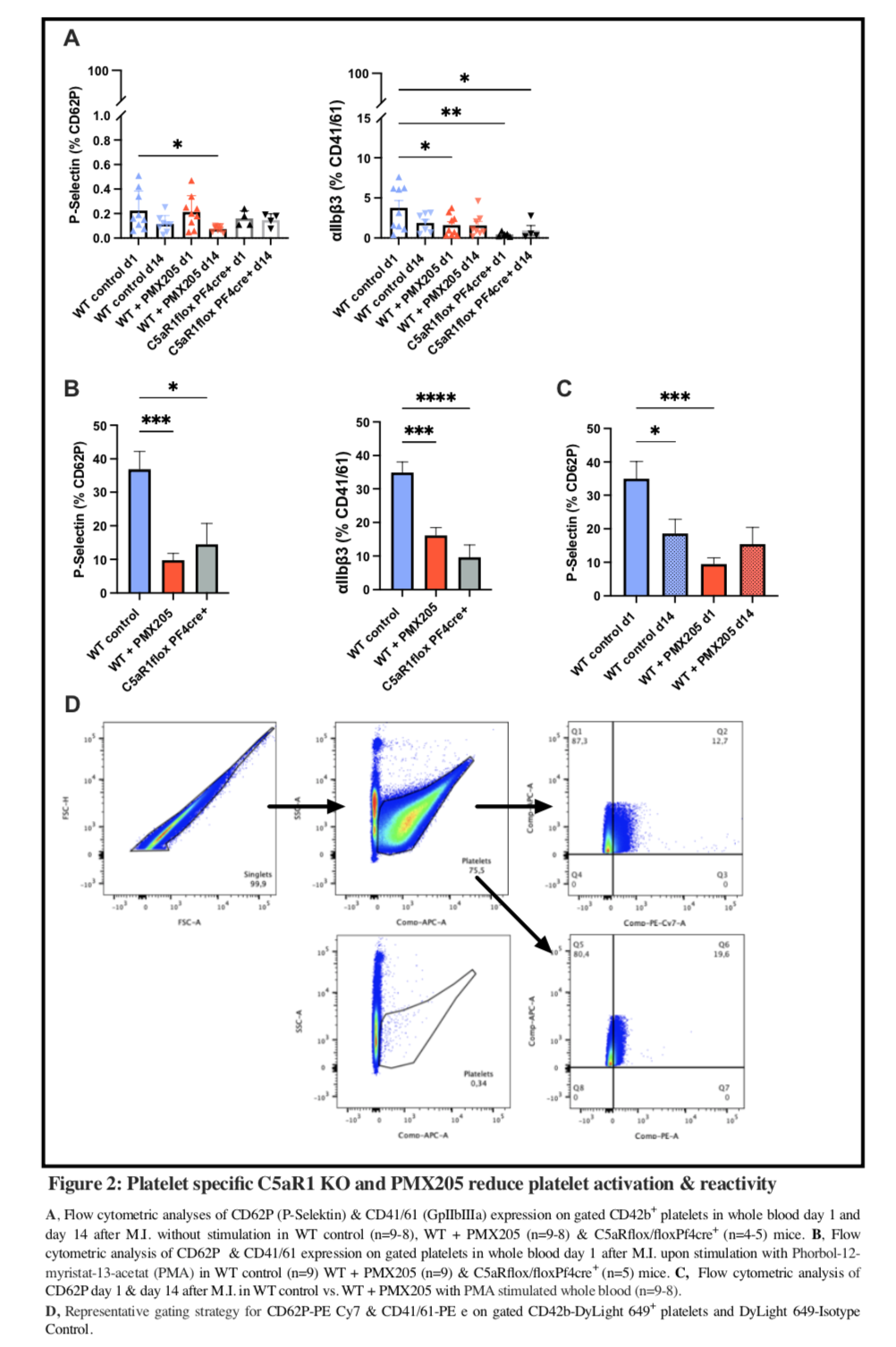
Methods: To study myocardial infarction (MI) and scar formation we applied the model of permanent LAD ligation to different groups of mice. 10–12-week-old C57BL/6 mice were treated with the C5aR1 antagonist PMX205 or PBS as control daily and 10–12-week-old C5aR1flox/floxPf4Cre+ (platelet specific C5aR1-/-) or C5aR1flox/floxPf4Cre- mice were injected with PBS subcutaneously daily and subjected to surgery four hours after initial injection. On day 1 and 13 after MI echocardiography was performed. On day 1 and 14 platelet-neutrophil complexes (PNCs; CD42b in Ly6G+) at baseline and surface expression of platelet activation markers (P-selectin, active GPIIb/IIIa) after stimulation with phorbol myristate acetate (PMA) were measured in a whole blood assay using flow cytometry. The mice were sacrificed on day 14 using the hearts for either histology or triphenyl tetrazolium chloride (TTC) staining to measure infarct size. Histology slices are analyzed using Mason’s Trichrome staining and immunofluorescence to stain for platelets, leukocytes and neovascularization markers.
Results: In PMX205-treated mice infarct sizes were significantly smaller at 3,5 mm and 2,0 mm distance from LAD ligation (3,5 mm: 31,49%±13,11 vs. 64.02%±9.81 of left ventricle; p<0,01, n=4; 2,0 mm: 31,73%±3,71 vs. 43,22%±7,75; p<0,05, n=4), Ejection Fraction (EF) on day 13 after MI was significantly higher (39,24%±6,64 vs. 28,31%±6,58, p<0,01), while EF was not significantly different on day one (41,07%±5,10 vs. 38,15%±4,79, p=0,628). Similarly, in platelet-C5aR1-/- mice infarct sizes were significantly smaller at 0,5 mm behind LAD ligation (12,92±2,38 vs. 25,24±3,38%;n=2 or 4, p<0,05) while EFs on day 13 (47,15%±7,45 vs 35,29%±15,99; n=4 or 3, p=0,256) and day one were numerically higher but not significantly different (47,09%±4,53 vs. 40,93%±6,46; n=4 or 3, p=0,59). PMA-induced platelet activation one day after MI was significantly lower in PMX205-treated and platelet-specific C5aR1-/- mice both for P-selectin expression (9.78%±6.11 vs. 36.76%±16.43, p<0.001, n=9 and 14.46%±13.96 vs. 36.76%±16.43 p<0.05, n=5) and GPIIb/IIIa activation (16.14%±7.03 vs. 34.84%±9.75, p<0.001, n=9 and 9.63±8.21 vs. 34.84±9.75, p<0.0001, n=5). PNCs were increased one day after MI across all groups and declined 14 days thereafter (WT + PMX205: 25.74%±11.29 vs. 4.96%±1.78, p<0.0001, n=9 or 8; WT control: 20.95%±8.02 vs. 6.15%±3.60 %, p<0.01, n=11 or 8; C5aR1flox/floxPf4cre+: 34.40%±13.84 vs. 5.06%±1.70, p<0.0001, n=5 or 4).
Conclusion: In conclusion, C5aR1 deficiency globally and platelet-specifically reduced MI injury and ameliorated heart function in a murine permanent LAD ligation model. In addition, platelet reactivity was reduced pointing towards a platelet specific C5aR1 contribution. Specific blockade of platelet C5aR1 is a potential target to mitigate myocardial infarction injury.
https://dgk.org/kongress_programme/ht2021/BS1021.htm